A Brief Introduction to Sodium Stearyl Fumarate
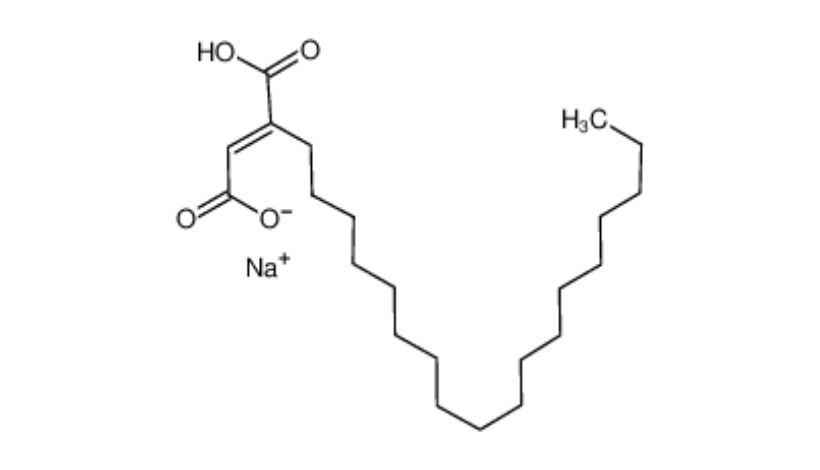
Molecular Formula:C22H39NaO4
Molecular Weight:390.53
CAS No.:4070-80-8
Appearance:White to off-white solid powder
Regular commercial package: 25kg/drum
What is Sodium Stearyl Fumarate?
- Abbreviated as SSF. It is a stable white powdery organic substance at room temperature and pressure, slightly soluble in methanol, and almost insoluble in water. Its melting point is 200-205℃, which is one of the indicators of product stability.
- The molecular structure of Sodium Stearyl Fumarate mainly consists of two parts: one part is the hydrophilic sodium fumarate salt, and the other part is the hydrophobic but lipophilic long-chain stearyl group.The amphiphilic molecular structure makes it perform better.
- Sodium Stearyl Fumarate is an organic salt formed by the isomerization of the reaction product of stearyl alcohol and maleic anhydride.
- It is an important excipient in the pharmaceutical and food industries.
Highassay’s Sodium Stearyl Fumarate
Highassay, as a supplier of Sodium Stearyl Fumarate from China, offers products that comply with various national pharmacopoeias and food standards. We provide data sheets such as COA and MSDS with our products.Whether you are in the pharmaceutical or food industry, we can supply Sodium Stearyl Fumarate raw materials that meet your requirements.
Highassay’s products not only meet standards but also offer exceptional usability, including features such as excellent lubrication and low inhibition; good compatibility; and stable physical properties, which will surely meet your production needs.Highassay has a long-term and stable supply capacity, can provide you with customized packaging solutions, and offers the necessary technical support.
For purchasing Sodium Stearyl Fumarate, choose Highassay.
Sodium Stearyl Fumarate(Pharmaceutical excipients-----Lubricant)
In the production of tablets and capsules, Sodium Stearyl Fumarate is added as an important excipient to form a hydrophobic film.It not only reduces friction between particles and improves fluidity, ensures the accuracy of drug dosage during manufacturing;but also reduce adhesion between materials and equipment, making it easier to demold tablets during production, reducing blockages, improving production efficiency, and ensuring a smooth and complete tablet surface.
.jpg)
.jpg)
Sodium Stearyl Fumarate(Food Industry - Additives)
Sodium Stearyl Fumarate is added to food as an emulsifier, stabilizer, and dough conditioner.It is mainly used in grain and starch-based products (bread, steamed buns, etc.), baked goods (cakes, biscuits, etc.), and powdered foods (to prevent caking).It can improve sensory qualities (fluffiness, softness), optimize the production process (easier processing), and extend shelf life.
The safety of Sodium Stearyl Fumarate
- Provided it is used in appropriate amounts, it is extremely safe as a pharmaceutical excipient and food additive, and is included in the pharmacopoeias of many countries; Sodium Stearyl Fumarate is an NF-grade product.
- As a physical lubricant that does not undergo chemical changes in the body, it is hardly absorbed and does not enter the bloodstream, and is eliminated from the body unchanged through feces.
- Sodium Stearyl Fumarate has a stable chemical structure and good compatibility with human tissues. At therapeutic doses, it does not cause irritation or damage to the gastrointestinal mucosa.


Sodium Stearyl Fumarate VS Magnesium Stearate
- Sodium Stearyl Fumarate is a high-performance lubricant option in modern solid dosage form manufacturing processes, while Magnesium Stearate is a traditional hydrophobic lubricant.
- Sodium Stearyl Fumarate has little effect on tablet disintegration or dissolution when exposed to water, forming a permeable, discontinuous hydrophilic-hydrophobic balanced membrane that does not affect drug release; whereas Magnesium Stearate severely hinders water penetration and tablet disintegration.
- Sodium Stearyl Fumarate is not hygroscopic, while Magnesium Stearate is hygroscopic. Its tendency to absorb moisture can lead to powder clumping, poor flowability, and degradation of the active pharmaceutical ingredient (API).
- If you have a sufficient budget and require a “safer, less side-effect-prone, and higher-quality” option, then Sodium Stearyl Fumarate is the preferred choice.
Specification of Sodium Stearyl Fumarate
- Specification of Sodium Stearyl Fumarate
| Items | Standards |
| Description | White or almost white powder |
| Identification | IR |
| Water (K.F. | 5.0% max |
| Lead | 0.001% max |
| Heavy metals | 20ppm max |
| Saponification value | 142.2~146.0 |
| Limit of sodium stearyl maleate and stearyl alcohol | |
| Sodium stearyl maleate | 0.25% max |
| Stearyl alcohol | 0.5% max |
| Organic volatile impurities | Meets the requirement |
| Residual solvents | Meets the requirement |
| Assay | 99.0~101.5% |
| Conclusion | Conforms to USP31/NF26 |
| Packaging and storage | Preserve in well-closed containers |
The amphiphilic nature of Sodium Stearyl Fumarate allows its molecules to be directionally adsorbed at the oil-water interface. By reducing interfacial tension, it forms a stable protective film, helping to evenly disperse the oil in the aqueous phase, thus forming a stable oil-in-water emulsion. It is used in salad dressings, cake batters, etc., ensuring both a good texture and preventing oil-water separation.
During the tablet compression process, its hydrophilic properties allow it to form a discontinuous and more permeable film, which allows water to penetrate, ensuring that tablet disintegration and drug release are not hindered. Simultaneously, its hydrophobic properties allow it to form a smooth film on metal or particle surfaces, giving the molecules lubricating characteristics.
The primary function of Sodium Stearyl Fumarate in cereal-based foods is to act as an anti-staling agent, extending the product’s freshness and shelf life. This is because Sodium Stearyl Fumarate strongly binds to starch molecules, effectively inhibiting the recrystallization process of starch.
Side effects of Sodium Stearyl Fumarate
1、Sodium Stearyl Fumarate, whether used as a pharmaceutical excipient or a food additive, will not cause significant harm to the human body within reasonable limits of addition, and is a relatively safe product ingredient.
2、Excessive addition of Sodium Stearyl Fumarate (>2%) can have some negative effects on the appearance and quality of the drug, such as:
- It can slow down the disintegration rate of tablets and the dissolution rate of the drug, affecting the efficacy of the medication.
- Reduced tablet strength results in tablets that are too fragile and easily broken, leading to substandard product quality and waste.
- If the mixture is not homogeneous, white powder will form spots on the surface of the tablets.
3、Excessive addition of Sodium Stearyl Fumarate (typically 0.2%-0.5% in flour) can have various negative effects on food, such as damaging the texture and taste of the food, interfering with processing, and violating food regulations.