A Brief Introduction to Phenolphthalein
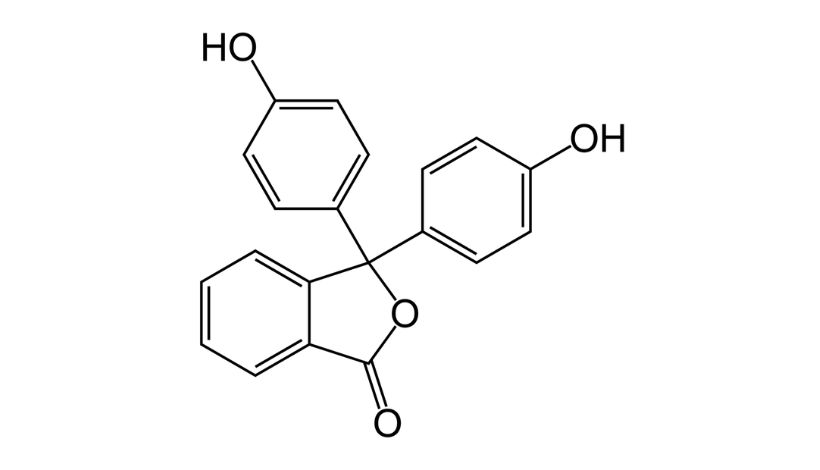
Molecular Formula:C20H14O4
Molecular Weight:318.323
CAS No.:77-09-8
Appearance:White to slightly yellow crystalline powder
Regular commercial package: 25kg/drum
What is Phenolphthalein?
Phenolphthalein is an organic compound that is relatively stable under normal temperature and pressure conditions. It is sparingly soluble in water and usually appears as a white or slightly yellowish crystalline powder. It is unstable under strongly acidic or strongly alkaline conditions and is readily soluble in ethanol.
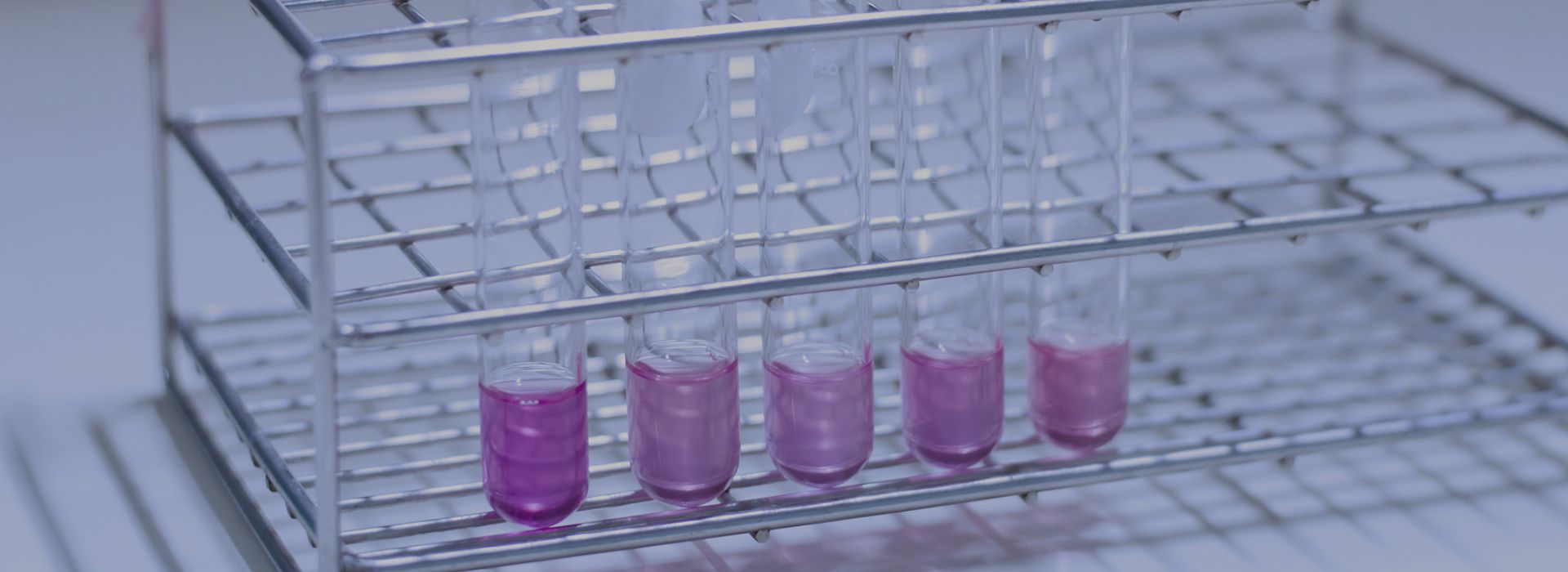
When Phenolphthalein encounters acidic or neutral solutions, no color change occurs, and the solution remains colorless; however, when Phenolphthalin encounters alkaline solutions, the solution turns purplish-red. This is why Phenolphthalein can be used as an indicator.
It is typically used in solutions prepared with alcohol, for testing pH levels. It is not only convenient to use but also highly safe.
Highassay’s Phenolphthalein
Highassay offers two grades of Phenolphthalein powder raw material: high-purity Phenolphthalein powder and lower-purity Phenolphthalein powder.
One is a high-purity white crystalline powder. High-purity Phenolphthalein is typically used in laboratories for analytical purposes or as an indicator because it possesses extremely stable chemical properties, and its color change range as an indicator is the most accurate.
Another type is Phenolphthalein with lower purity (containing impurities, such as substances similar to fluorescein) or that has undergone partial degradation (due to oxidation from air and light exposure over time). It typically appears as a pale yellow, light yellow, or off-white powder or crystals. Although the accuracy might be slightly compromised, it is generally acceptable for most experiments.
As a controlled chemical substance, we provide an MSDS (Material Safety Data Sheet) with the product; please use it according to regulations. If you need to purchase Phenolphthalein raw materials, please choose Highassay; we will provide you with comprehensive service throughout the process.
How does Phenolphthalein work?
The molecular structure of Phenolphthalein, and how it changes in acidic and alkaline environments, determines the color of the Phenolphthalein indicator.At different pH values, Phenolphthalein loses or gains protons (H⁺), altering its structure. These different structures absorb light differently, thus exhibiting different colors.
For example, in neutral and acidic environments, the Phenolphthalein molecule is in a closed-ring state, absorbing little to no visible light, thus the solution appears colorless; in a weakly alkaline environment, the cyclic structure opens, and after absorbing blue-green light, the solution turns red; in a strongly alkaline environment, the structure of the Phenolphthalein molecule is destroyed by an excess of hydroxide ions, undergoing an irreversible chemical reaction, and therefore no longer absorbs visible light, causing the solution to become colorless again.


Uses of Phenolphthalein: Reagent, Intermediate
- Chemical analysis uses: As an acid-base indicator, Phenolphthalein is primarily used to test for bases, because its pH range is 8.2-10.0. At pH < 8.2, the solution is colorless; at pH > 8.2, the solution turns red. When the pH exceeds 10, the solution gradually fades, becoming colorless again as the alkalinity increases.
- Industrial uses: Used in plastic synthesis. It should be noted that this is not a raw material for the synthesis of ordinary plastics, but rather a key intermediate for high-end engineering plastics (a crucial starting material for polyaryletherketone/sulfone polymers containing a diazaphthalenone structure), widely used in high-value-added specialty materials for cutting-edge technology and industrial fields. This material possesses excellent heat resistance, water resistance, chemical corrosion resistance, heat aging resistance, and good processability.
Specific Applications of Phenolphthalein Indicator
- Textile Industry: Used to measure the pH of fabrics during the dyeing and printing process. For example, excessive alkaline components on mercerized fabric can affect the fabric’s luster.
- Laboratory: In schools, laboratories, and other settings, it is used in experiments such as acid-base titrations and redox titrations, commonly known as Phenolphthalein T The endpoint of the acid-base titration can be determined by observing the color change.
- Fun Experiment: “Invisible Ink” – Writing with Phenolphthalein solution results in invisible writing after drying. When sprayed with an alkaline solution such as baking soda solution, the writing reappears and turns pink.
- Medical applications: In vitro diagnostic reagents, which detect alkaline changes in physiological or pathological conditions by measuring the pH value of urine or other body fluids. Ingestion is strictly prohibited, as Phenolphthalein is carcinogenic when taken internally.

Specification of Phenolphthalein
- Specification of Phenolphthalein
| Product Name | Phenolphthalein |
| Structure | (HO-C₆H₄)₂C(C₆H₄O₂) |
| boiling point | 557.7 ℃ |
| Melting point | 258-263 ℃ |
| Density | 1.299 g/cm³ |
| Flash point | 24℃ |
| Molar mass | 318.323g/mol |
| Solubility | Soluble in ethanol and alkaline solutions, insoluble in water |
| Package | Regular commercial package: 25kg/drum |
| Storage | Store in a sealed, dry, and dark place, and keep separately |
Phenolphthalein indicator is a chemical reagent that changes color in different acidic and alkaline environments. Because Phenolphthalein is not easily soluble in water, Phenolphthalein indicator is usually prepared by mixing Phenolphthalein with alcohol.
Phenolphthalein Titration is the most commonly used indicator choice in acid-base titrations, where the reaction endpoint is determined by observing the change in the solution’s color.
Phenolphthalein raw material should be stored in a sealed, dry, and light-protected container, separately from other substances. Pure Phenolphthalein powder is chemically stable and has a shelf life of 3-5 years or even longer.
Dangers of Phenolphthalein
- Phenolphthalein, the raw material, is a toxic chemical with a very high risk factor and is carcinogenic; therefore, it must be handled with extreme caution during operation.
- Phenolphthalein was once used to treat chronic constipation, but due to its carcinogenic properties, it has been replaced by other products and has gradually disappeared from the market.
- The Phenolphthalein indicator solution has a very low concentration of Phenolphthalein, being a 0.5%-1% alcoholic solution, which reduces the risk, but ingestion and contact with the eyes should still be avoided.