What are steroids?
Steroids are a class of chemical substances with a steroidal structure that are required for biological activities in the human body. They are primarily secreted by the adrenal glands and gonads. Steroids are a class of organic compounds composed of four rings and a short chain.
Steroids can be divided into two categories: endogenous steroids and exogenous steroids.

Endogenous steroids are hormones secreted by the human body, such as adrenaline, sex hormones, corticosteroids, and progesterone. Under normal circumstances, these hormones are self-regulated by the body to enhance adaptability and adjust metabolic balance, among other physiological processes. Exogenous steroids generally refer to steroid drugs, also known as steroid hormones. They are synthetic compounds that exhibit biological activities similar to endogenous steroids.
Cholesterol and its derivatives
Cholesterol is a key component of the biological membranes of higher animals, particularly abundant in nervous tissue and the adrenal glands. It accounts for 17% of brain solid matter, over 20% of plasma membrane lipids, and 5% of organelle membranes.
Its molecular shape differs from other membrane lipids: the polar head is a hydroxyl group at the 3rd position, and the hydrophobic tail consists of four rings and three side chains. This unique structure enables biological membranes to maintain a relatively stable liquid crystal state over a wide temperature range, providing suitable fluidity. The hydroxyl group at the 3rd position of cholesterol can form esters with higher fatty acids (primarily linoleic acid), which is the storage and transport form of cholesterol.
Cholesterol is also a precursor of several active substances. Steroid hormones, vitamin D3, and bile acids are all cholesterol derivatives.
Bile acids are synthesized in the liver, with side chains oxidatively broken down to form valeric acid. Because bile acids contain carboxyl groups and multiple hydroxyl groups, all located on one side of the ring, they possess both hydrophilic and hydrophobic sides. They are highly effective emulsifiers, promoting the dispersion of oils and fats, making them easier to digest and absorb. Human bile contains three types of bile acids: cholic acid, deoxycholic acid, and chenodeoxycholic acid. These acids, which can bind to glycine or taurine, are the primary cause of bile’s bitterness. Bile acids also inhibit the formation of cholesterol stones in bile and regulate metabolism and immunity.
What do steroids do?
Our bodies produce these essential steroid hormones to maintain good health.

Steroid hormones primarily fall into two categories: sex hormones and adrenocortical hormones.
Sex hormones promote the development of sexual organs and maintain secondary sexual characteristics.
Adrenocortical hormones are involved in numerous physiological processes, including regulating sugar, fat, and protein metabolism, maintaining stress responses, immune responses, and controlling inflammation. They can also inhibit and reduce inflammatory symptoms such as swelling, redness, itching, and allergic reactions.
Anabolic steroids
Anabolic steroids are a class of chemically synthesized derivatives that are similar in structure and activity to the human male hormone testosterone. They promote skeletal muscle growth and enhance male characteristics. Anabolic steroids share a steroidal chemical structure similar to testosterone. Common examples include stanozolol, trenbolone, oxandrolone, oxymetholone, and methandrostenolone. These products were originally synthesized to treat hypogonadism, but were later found to have the side effect of promoting muscle growth. They were widely abused by athletes and are now classified as performance-enhancing drugs.
Anabolic steroids side effects
Steroid abuse has many negative consequences.
It disrupts the body’s natural hormonal system.
Disrupts the skeletal muscle growth system
Damages the cardiovascular system
Adversely affects the liver
Also has side effects on the skin
Can easily cause irritability and violent tendencies in users
Contact dermatitis topical steroids
Contact dermatitis is divided into irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD).
Small lesions of allergic contact dermatitis can be treated with topical corticosteroids or topical calcineurin inhibitors. Symptomatic treatments such as topical calamine lotion can also help relieve symptoms. For patients with large lesions (greater than 20%) or those involving the face, hands, feet, or genitals who desire rapid symptom relief (e.g., eyelid involvement) or who are unresponsive to topical medications, oral corticosteroids may be an option.
Topical steroids relieve dermatitis by suppressing inflammation and reducing itching and pruritus. The appropriate topical steroid can be selected based on the affected area; please refer to the following instructions.
Weak-strength: Hydrocortisone (0.1%-2.5%) and desonide are suitable for the face, delicate skin, or children.
Medium-strength: Triamcinolone acetonide and mometasone furoate are used for mild to moderate inflammation on the trunk or extremities.
Strong-strength: Clobetasol propionate and halcinonide are limited to short-term use for stubborn lesions (such as on the palms and soles).
What are nonsteroidal anti-inflammatory drugs (NSAIDs)?
Drugs with anti-inflammatory properties can be mainly divided into two types: steroids and nonsteroidal anti-inflammatory drugs (NSAIDs).
The two have completely different mechanisms of action.
Steroids are primarily used to control autoimmune diseases. Because some patients may resist them or have more side effects, they should only be prescribed by a doctor after evaluating the patient’s condition.
NSAIDs primarily work by interfering with the body’s cyclooxygenase (COX) enzyme, hindering the production of prostaglandins, which in turn affects the cellular responses and pathogenesis of prostaglandins. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for fever reduction, analgesia, and inflammation. They are categorized into two types based on their mechanism of action: non-selective NSAIDs that inhibit both COX-1 and COX-2, and COX-2 inhibitors, which have a lower risk of gastric bleeding.
Based on their chemical structure, NSAIDs can be divided into formic acid, acetic acid, propionic acid, oxicams, coxibs, and pyrazolones.
NSAIDs primarily include aspirin, ibuprofen, indomethacin, diclofenac, naproxen, acetaminophen, and rofecoxib. Although some NSAIDs are available over the counter, they also have side effects. Common side effects include gastrointestinal discomfort and edema. Long-term use at high doses may cause adverse reactions such as gastric ulcers, internal bleeding, and heart problems. They may also increase the risk of chronic kidney disease. People with liver, heart, or kidney disease should be particularly aware of side effects. For long-term use, consult a healthcare provider for advice on proper use.
What are the topical steroids?
Topical steroids are available in a wide variety of types.
Topical steroids are categorized according to their potency.
Please select the appropriate product based on your specific needs.
Weak-potency
0.05% desonide ointment, cream, gel, foam, and lotion
0.01% fluocinolone cream and 0.05% fluocinolone solution
0.025% triamcinolone acetonide cream and solution
0.5% prednisone acetate ointment
0.05% dexamethasone acetate ointment
Intermediate-potency
0.1% mometasone furoate cream and lotion
0.1% hydrocortisone butyrate ointment, cream, and lotion
0.1% triamcinolone acetonide cream, ointment, and lotion
Strong-potency
0.05% betamethasone dipropionate Gels and Ointments
Beclomethasone Dipropionate 0.025% Ointment
Halometasone 0.05% Cream
Fluocinolone Acetate 0.05% Ointment, Cream, Gel, and Solution
Triamcinolone Acetonide 0.1% Ointment, Triamcinolone Acetonide 0.5% Cream
Mometasone Furoate 0.1% Ointment
Super Strength
Fluocinolone Acetate 0.1% Cream
Diflorasone Acetate 0.05% Ointment
Clobetasol Propionate 0.05% Gel, Ointment, Cream, and Foam
Depend on the location and type of skin disease, refer to the following usage instructions.
Weak: Facial dermatitis, eyelid dermatitis, diaper dermatitis, and perianal eczema.
Moderate: Atopic dermatitis, seborrheic dermatitis, vulvar lichen sclerosus, pruritus anus, acute radiation dermatitis, severe intertriginous rash, scabies (after scabicide treatment), and stasis dermatitis.
Strong/super-strong: Alopecia areata, recalcitrant atopic dermatitis, discoid lupus erythematosus, chronic eczema, lichen planus, lichen sclerosus, chronic simplex lichen, nummular eczema, psoriasis, bullous pemphigoid, localized vitiligo, and severe hand eczema.
Select the appropriate dosage form based on the nature of the lesion.
Ointment: Hypertrophic, keratinized, and desquamative lesions, especially on the palms and soles.
Cream and gel: Acute, subacute, and chronic lesions.
Gel, lotion, and solution: Scalp and areas with dense hair.
Tincture and vinegar: Hypertrophic and lichenified lesions.
Nonsteroidal anti-inflammatory drugs (NSAIDs) for rheumatoid arthritis
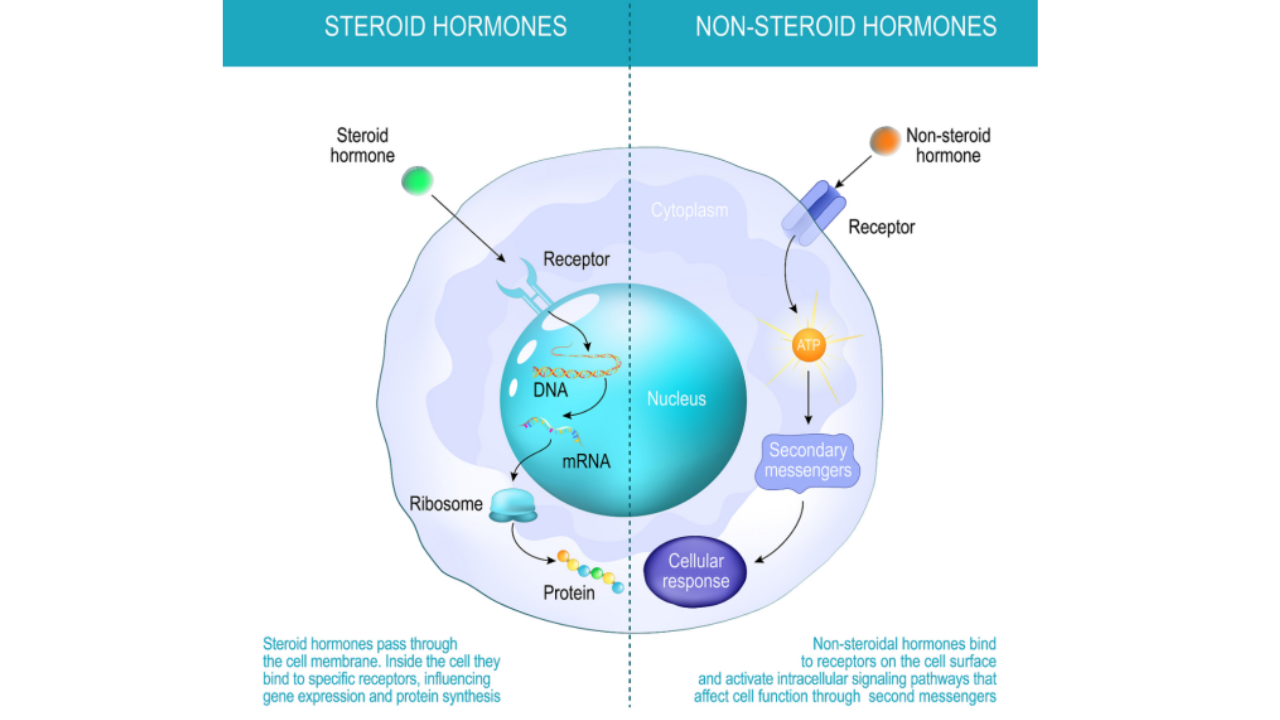
NSAIDs such as ibuprofen extended-release capsules, diclofenac sodium enteric-coated tablets, and celecoxib capsules can relieve pain and inflammation.
Side effects of injection of steroids
Infection
Allergic reaction
Bleeding into joints
Tendon rupture
Skin discoloration
Weakening of bones, ligaments, and tendons (due to frequent, repeated injections into the same area)
Oral steroids
Corticosteroids come in various dosage forms, including oral formulations (such as tablets, capsules, and solutions). Common oral corticosteroids include prednisolone, prednisone, dexamethasone, betamethasone, and hydrocortisone. Prednisolone is the most commonly used oral corticosteroid.
Natural steroids
Corticosteroids, produced by the adrenal cortex, are natural hormones. Under normal circumstances, our bodies produce these essential hormones to maintain health. Corticosteroid secretion is involved in numerous physiological responses, including stress response, immune response, and inflammation control. They can suppress and reduce inflammatory symptoms such as swelling, redness, itching, and allergic reactions.
Medical medications are generally synthetic analogs of these hormones, primarily used to treat various inflammatory and immune system-related diseases.
Are peptide steroids?
Peptides are not the same as hormones, but some hormones belong to the peptide class. Hormones are signaling molecules that regulate physiological activities in organisms. Based on their chemical structure, they can be divided into steroid hormones (such as sex hormones), amino acid derivatives (such as thyroid hormones), and peptide/protein hormones (such as insulin). Therefore, peptides can be a type of hormone, but not all peptides are hormones.
Types of steroid hormones
They primarily include sex hormones and adrenal cortex hormones.
Sex hormones are steroid hormones secreted by the gonads (testicles and ovaries) and adrenal glands in the human body. They are primarily involved in the development of the reproductive system, maintaining sexual function, and forming secondary sex characteristics. They regulate human physiology and influence various functions, including growth, metabolism, and mood. Sex hormones include estrogen, progesterone, and androgen.
Estrogens include Estradiol, Estradiol Valerate, Estradiol Benzoate, Estradiol Enanthate, Estriol, Ethinylestradiol, and Estradiol Cypionate.
Androgens include Testosterone Cypionate, Testosterone Undecanoate, Testosterone Dodecanoate, Testosterone Tridecanoate, Testosterone Propionate, Testosterone, Testosterone Enanthate
Progesterone includes Cyproterone Acetate, Mifepristone, Progesterone, Norethindrone/Norethisterone, Norethisterone Enanthate, Norethisterone Enanthate, Medroxyprogesterone Acetate, Megestrol Acetate, Estrone
Corticosteroids include Prednisolone, Prednisone Acetate, Prednisolone 21-mesyl, Prednisolone Acetate, Hydrocortisone Butyrate, Mometasone Furoate, Fluticasone Propionate, Betamethasone Valerate, Betamethasone Dipropionate, Dexamethasone Acetate, Dexamethasone Sodium Phosphate, Prednisolone Sodium Phosphate, Betamethasone Sodium Phosphate, Betamethasone, Dexamethasone, Triamcinolone Acetonide Acetate, Triamcinolone Acetonide, Prednisone, Methylprednisolone Acetate, Methylprednisolone, Hydrocortisone Acetate, Hydrocortisone, Flumethasone, Methylprednisolone Hemisuccinate, Ciclesonide, Eplerenone
Corticosteroids are the most potent anti-inflammatory drugs available. They are synthetic substances with the same effects as cortisone. Cortisone is naturally produced in the human body by the adrenal cortex. Later, humans artificially synthesized many corticosteroids that were more potent and lasted longer than natural cortisone to meet medical needs. Common corticosteroids include Prednisolone, Prednisone Acetate, Prednisolone 21-mesyl, Prednisolone Acetate, Hydrocortisone Butyrate, Mometasone Furoate, Fluticasone Propionate, Betamethasone Valerate, Betamethasone Dipropionate, Dexamethasone Acetate, Dexamethasone Sodium Phosphate, Prednisolone Sodium Phosphate, Betamethasone Sodium Phosphate, Betamethasone, Dexamethasone, Triamcinolone Acetonide Acetate, Triamcinolone Acetonide, Prednisone, Methylprednisolone Acetate, Methylprednisolone, Hydrocortisone Acetate, Hydrocortisone, Flumethasone, Methylprednisolone Hemisuccinate,Ciclesonide, Eplerenone.
They can be applied directly to various areas of inflammation, providing rapid anti-inflammatory effects. Examples include ointments that can be applied directly to affected skin areas, eye drops that can be placed directly into the eyes, and nasal sprays that can be administered directly into the nasal cavity to treat asthma. They can also be injected into joints for anti-inflammatory effects in rheumatoid arthritis.
Synthesis and Mechanism of Action of Steroid Hormones
The glucocorticoid receptor (GR or GCCR) is a typical nuclear hormone receptor, encoded by the NR3C1 (nuclear receptor subfamily 3 group C member 1) gene. The corresponding HRE, also known as the GRE, has the sequence GGAACAnnnTGTTCT. GR also has coactivators, such as the SRC (steroid receptor coactivator) protein. In the liver, two major target genes of cortisol are the gluconeogenic genes PCK1 (cytoplasmic phosphoenolpyruvate carboxykinase) and G6PC (glucose-6-phosphatase). Maximal expression of both PCK1 and G6PC requires other factors, including the nuclear receptor PPARα. Therefore, the PPARα gene, encoding PPARα, is also a target of cortisol activation. In adipose tissue, GR promotes lipolysis by activating HSL expression and also promotes the differentiation and maturation of preadipocytes. In skeletal muscle, glucocorticoids promote glycogen and protein hydrolysis, and the amino acid carbon skeletons serve as raw materials for hepatic gluconeogenesis. Mineralocorticoids, primarily aldosterone, primarily function to conserve sodium and excrete potassium, accompanied by chloride and water absorption, thus increasing blood pressure. This mechanism is through the mineralocorticoid receptor (MR) inducing the expression of the sodium pump (sodium-potassium ATPase), the epithelial sodium channel (ENaC), and the Na+-Cl- cotransporter (NCC).
Sex hormones and adrenocortical hormones are both cholesterol derivatives and lipid-soluble, allowing them to cross the plasma membrane and bind to intracellular receptors (also known as nuclear receptors, NRs) to regulate gene expression. Steroid hormone synthesis typically occurs in the adrenal cortex, gonads (testes and ovaries), brain, placenta, and adipose tissue. Two major enzymes are involved in this synthesis: cytochrome P450 enzymes (CYPs) and hydroxysteroid dehydrogenases (HSDs). The first step in steroid hormone synthesis is the cleavage of six carbon atoms from the side chain of cholesterol, producing pregnenolone. This is the rate-limiting step in this three-step pathway. In humans, it is catalyzed by cholesterol-20,22-desmolase (also known as CYP11A or P450ssc).
This enzyme is bound to the inner mitochondrial membrane in all steroidogenic tissues, necessitating the transport of cholesterol into the mitochondria. This transport process, mediated by the steroidogenesis rapid regulatory protein (StAR) on the outer membrane, is the rate-limiting step in this process. Adrenocorticotropic hormone (ACTH) elevates cAMP, activating PKA, which phosphorylates and activates cholesterol ester hydrolase and StAR, thereby promoting corticosteroid synthesis.
Steroid Derivative Vitamin D
Vitamin D, also known as calciferol, is derived from a cyclopentahydrophenanthrene ring that is opened. Vitamin D can be taken directly or converted from provitamin D via ultraviolet radiation. Ergosterol in vegetable oils and yeast is converted to D2 (ergocalciferol), while 7-dehydrocholesterol in the skin of animals is converted to D3 (cholecalciferol). German chemist Adolf Windaus won the 1928 Nobel Prize in Chemistry for his research on sterols and vitamin D.
The conversion reaction to provitamin D requires light with a wavelength of 270-300 nanometers (UV-B), which is blocked by glass or sunscreen. Therefore, sunbathing behind glass or with sunscreen is ineffective. For birds and fur-bearing mammals, fur or feathers block UV rays from reaching the skin. Oily secretions produced by their skin adhere to the surface of feathers or fur. When exposed to UV rays, they produce vitamin D, which is absorbed orally during preening.
The physiological function of vitamin D is primarily related to bone calcification. Calcification requires sufficient calcium and phosphorus, with a ratio of between 1:1 and 2:1, as well as the vitamin D derivative calcitriol. Vitamin D3 is first hydroxylated in the liver to form 25-hydroxyvitamin D3, which is then further hydroxylated in the kidneys to form 1,25-dihydroxyvitamin D3 (also known as calcitriol, calcitriol, or calcitriol). This second hydroxylation is strictly regulated, normally producing only the inactive 24-hydroxylated product. Only when blood calcium is low does parathyroid hormone secretion activate the 1-hydroxylase.
Vitamin D deficiency can lead to rickets in infants, children, and adolescents, manifesting as impaired calcium and phosphorus metabolism, incomplete bone calcification, growth retardation, and muscle cramps. Adults who inadequate dietary vitamin D and lack ultraviolet exposure are more susceptible to fractures. Hepatobiliary disease, kidney disease, or certain medications can also inhibit vitamin D hydroxylation, leading to similar symptoms.
Because fat-soluble vitamins tend to accumulate in the body, excessive intake of vitamin D can also cause toxicity, leading to migratory calcification and calcification of the kidneys, heart, pancreas, uterus, and synovial mucin. High blood calcium levels can also lead to kidney stones, while bones become loose and softened due to calcium withdrawal. So, don’t blindly supplement with vitamin D.
Calcitriol
Calcitriol is a hormone secreted by the renal cortex that regulates blood calcium and phosphate concentrations, promoting healthy bone growth. Bone growth is a complex process involving the proliferation and differentiation of various cells, including osteoprogenitors, osteoblasts, osteoclasts, chondroblasts, and chondrocytes, as well as the formation of different bone morphologies, including osteoid, woven, and lamellar bone. Calcitriol is involved in many of these processes, including regulating gene expression and signaling pathways.
Calcitriol also acts on intestinal mucosal cells, activating the synthesis of calcium-binding proteins through vitamin D receptors, thereby promoting intestinal absorption of calcium and phosphate. In the kidneys, calcitriol promotes calcium and phosphate reabsorption. Calcitriol also regulates bone development by inhibiting parathyroid hormone (PTH) secretion and promoting the synthesis of fibroblast growth factor 23.
Calcitriol has effects beyond bone development. It has antiproliferative and prodifferentiation effects on most cells. This makes it potentially useful in the prevention and treatment of malignant tumors, but its specific clinical effectiveness remains controversial. Calcitriol is also important for the immune system, including both adaptive and innate immunity. This is related to its regulation of cell proliferation and differentiation. In this respect, vitamin D is somewhat similar to vitamin A, as they share similar receptor types.
Are steroids illegal?
It must be noted that steroids are illegal in most places.
A note from Highassay.
Highassay is a professional supplier of hormone raw materials. Our products comply with GMP standards, have low impurity content, and comply with USP/EP/BP. We manufacture them commercially and offer competitive pricing. They are suitable for both laboratory use and pharmaceutical production. Please contact us for inquiries.