Glycosylation and Glycation
This article introduces protein glycation and glycosylation, focusing on N-glycosylation.
Among the various post-translational modifications of proteins, glycosylation is a very important one. Under enzyme catalysis, sugar groups form covalent bonds, typically glycosidic bonds, with certain residues on the protein. This should be distinguished from glycation. In conditions such as diabetes, glucose can spontaneously react with lysine in proteins to form a Schiff base, which then rearranges to produce a glycated protein. This can then undergo further reactions to produce more complex derivatives.
Thus, after glycation, the sugar group becomes a derivative, while after glycosylation, the sugar group remains intact. In a broad sense, glycation includes all reactions that attach sugars to proteins, including glycosylation. However, it is now generally used to specifically refer to the non-enzymatic reactions mentioned above. Highly hydrophilic sugar groups can significantly influence the physicochemical properties and biological functions of proteins. The diverse structures of monosaccharides and sugar chains make them suitable as labels, making glycosylation very common in organisms. Approximately 50% of all known proteins are glycosylated, and at least 1% of the genes in the human genome are involved in glycan biosynthesis. It is estimated that mammalian sugar chains have over 7,000 structures, with approximately 10 monosaccharides (including sugar derivatives) as their structural monomers: glucose (Glc), galactose (Gal), mannose (MAN), xylose (Xyl), fucose (Fuc), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), glucuronic acid (GlcA), iduronic acid (IDOA), and sialic acid (SA). Two of these uronic acids are primarily found in proteoglycans.
Among basic amino acids, at least nine can be modified with sugar groups. Glycosyl groups can be attached to proteins in various ways, such as through amide bonds to Asn side chains (N-glycosylation), through glycosidic bonds to the hydroxyl groups of Ser, Thr, hydroxylysine (collagen), or Tyr (glycogen) (O-glycosylation), or through C-C bonds to the C2 position of Trp (C-mannosylation). Alternatively, glycosyl groups can be attached to proteins indirectly via glycosylphosphatidylinositol (GPI) anchors.
In eukaryotes, the vast majority of protein glycosylation occurs along the secretory pathway, beginning in the endoplasmic reticulum and concluding in the Golgi apparatus. The resulting modifications can affect protein localization; for example, phosphorylation of mannose in the Golgi apparatus diverts proteins from their default secretory to lysosomal pathways.
N-glycosylation
N-glycosylation is the most prevalent form of glycosylation in eukaryotes and has been the most extensively studied. Eukaryotic cells typically undergo core glycosylation in the endoplasmic reticulum (ER). This involves the transfer of a presynthesized sugar chain (core sugar chain) to an Asn in the Asn-X-(Ser/Thr) sequence of a target protein, catalyzed by oligosaccharyltransferase (OST), where X represents any amino acid except Pro.
The core sugar chain is carried by dolichol, known as a lipid-linked oligosaccharide (LLO). Dolichol is synthesized from the cholesterol synthesis intermediates IPP and FPP. It is phosphorylated with CTP by dolichol kinase (DOLK) to produce dolichol phosphate (Dol-P).
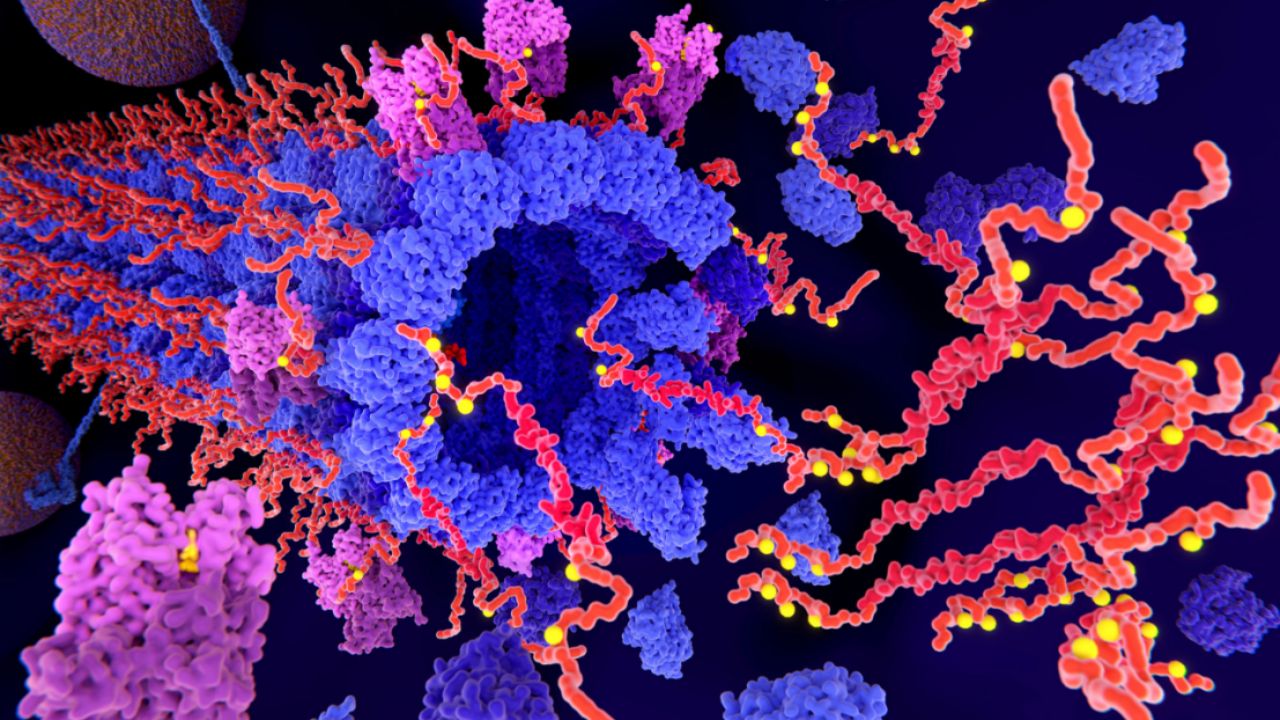
On the cytoplasmic face of the ER membrane, dolichol phosphate reacts with UDP-GlcNAc to form a GlcNAc-PP-Dol structure. This structure then reacts sequentially with UDP-GlcNAc and five GDP-Man residues to form a Man5 GlcNAc2-PP-Dol structure. This structure flips to the lumen of the endoplasmic reticulum, where four mannose and three glucose residues are added to form LLO, with the structure Glc3Man9GlcNAc2-PP-Dol.
The LLO sugar chain is transferred by OST to the Asn-X-(Ser/Thr) sequence of the nascent peptide chain (in rare cases, it can be ASN-X-Cys or other non-standard sequences). Strictly speaking, this step is primarily co-translational glycosylation, with post-translational glycosylation occurring in rare cases. Literature suggests that when the acceptor peptide is NXS, the glycosylation efficiency is 40-fold lower than when it is NXT, making post-translational glycosylation more likely.
After the core sugar chain is transferred, some modifications are required, primarily the removal of the terminal glucose. The protein then enters the Golgi apparatus for terminal glycosylation, which involves removing some mannose residues and adding other sugars. After terminal glycosylation, N-glycosylated proteins are mainly divided into three types: high mannose type containing all 9 mannose groups, hybrid type containing other sugar groups, and complex type containing sialic acid.
O-Glycosylation
O-Glycosylation is also a common post-translational modification, forming an O-glycosidic bond between a sugar group and an amino acid side chain hydroxyl group in a peptide chain. The hydroxyl groups available for bond formation are primarily the alcoholic hydroxyl groups of serine and threonine, but also include the hydroxyl group of hydroxylysine (e.g., collagen) and the phenolic hydroxyl group of tyrosine (e.g., glycogen).
N-glycosylation involves presynthesis of a core sugar chain and then its entirety being transferred to the acceptor peptide; O-glycosylation typically involves the individual transfer of activated sugar groups to the corresponding residues. Therefore, N-glycosylation requires the use of oligosaccharyltransferases (oligosaccharyltransferases), while O-glycosylation utilizes various glycosyltransferases (GTs).
GTs serve as substrates for various activated sugar groups. Different sugar groups are activated in different ways, but all are linked to nucleotides. The activation carrier for glucose and galactose is UDP, for mannose and fucose is GDP, and for sialic acid is CMP. The activation process typically occurs in the cytoplasm, after which the corresponding transport proteins transport the products to the endoplasmic reticulum or Golgi apparatus. Within the endoplasmic reticulum, there is also a transport pathway that relies on dolichol phosphate.
The Golgi apparatus contains a variety of GTs and glycosidases that modify N- and O-glycans. These enzymes are distributed in different compartments, and proteins undergo different modifications sequentially during their transport. The Golgi membrane is dynamically recycled, with both anterograde and retrograde vesicular transport. Therefore, the distribution of enzymes is also dynamic, sometimes overlapping, and some enzymes also recycle to the endoplasmic reticulum.
O-glycosylation is generally classified based on the first sugar group attached to the peptide chain. There are seven major types of O-glycans in the human body, the most common of which is the mucin-type, with the first sugar group being N-acetylgalactosamine (-GalNAc-1–Ser/Thr).
Mucin, also known as mucin, is abundant in mucus secreted in the digestive, respiratory, and reproductive tracts. The large number of short-chain O-glycans in mucins contributes to their viscosity in solutions. For example, the mucosal surface of the stomach and intestines contains a layer of mucin gel that forms a physical barrier, shielding the mucosal surface from digestive juices and degradation.
Most O-glycosylation begins in the endoplasmic reticulum, but mucin-specific O-glycosylation begins in the Golgi apparatus. The human Golgi apparatus contains 20 N-acetylgalactosamine transferases (GalNAc-T), with overlapping functions but subtle differences in substrate specificity. This results in a mild phenotype caused by deficiency in a particular enzyme, in stark contrast to the severe consequences of deficiency in other glycosylation enzymes.
After the introduction of the first sugar group, other glycosyltransferases can continue to add sugar groups, extending and branching the sugar chain to form various types of sugar chains. Depending on the type and attachment method of the second sugar group, mucin sugar chains can be further divided into eight core structures, each with distinct tissue specificities and physiological functions.
For mucins, the addition of sialic acid, fucose, and other enzymes serves as termination signals. For example, premature sialylation of the first GalNAc residue by ST6GalNAc-1 leads to the cancer-associated structure STn.
 O-linked galactose occurs primarily in collagen, attached to hydroxylysine. Lysyl hydroxylase (EC 1.14.11.4) catalyzes the hydroxylation of lysine in the -X-Lys-Gly- sequence, a reaction that requires divalent iron, α-ketoglutarate, oxygen, and ascorbic acid.
O-linked galactose occurs primarily in collagen, attached to hydroxylysine. Lysyl hydroxylase (EC 1.14.11.4) catalyzes the hydroxylation of lysine in the -X-Lys-Gly- sequence, a reaction that requires divalent iron, α-ketoglutarate, oxygen, and ascorbic acid.
Some hydroxylysine residues within the helical domain of collagen can undergo O-glycosylation to produce G-Hyl (galactosylhydroxylysine) and GG-Hyl (glucosylgalactosylhydroxylysine). These reactions are catalyzed by GT (EC 2.4.1.50) and GGT (EC 2.4.1.66), respectively, using UDP-activated monosaccharides as donors. These reactions all occur in the endoplasmic reticulum, before collagen triple helix formation. This is the polar opposite of mucins. Glycosylation of hydroxylysine is associated with bone calcification, and defects in glycosylation can lead to diseases such as osteogenesis imperfecta and osteoporosis. Glycosylation is also believed to be linked to biological processes such as collagen cross-linking, remodeling, and collagen-cell interactions. The specifics of collagen glycosylation vary greatly across different tissues and physiological and pathological conditions, and many mechanisms remain to be elucidated.
O-GlcNAc modification
O-GlcNAc modification is involved in diverse metabolic and gene expression regulation, and is associated with numerous important physiological processes.
O-GlcNAc modification of serine and threonine by β-N-acetylglucosamine occurs in a variety of nuclear and cytoplasmic proteins, including transcription factors, cytoskeletal proteins, oncogenes, and kinases. To date, over 600 proteins have been identified as being modified with O-GlcNAc. O-GlcNAc-modified proteins are involved in biological processes including nucleotide metabolism (e.g., thymidine kinase), sugar metabolism (e.g., glucose-6-phosphatase), metabolic regulation (e.g., AMPK), cell growth and maintenance (e.g., MYC, Sp1), DNA damage response, intracellular transport, transcription (e.g., RNA polymerase II), and translation (e.g., eIF-5).
O-GlcNAc synthesis occurs through the hexosamine biosynthetic pathway (HBP), which utilizes glucose, acetyl-CoA, glutamine, and UTP to produce UDP-GlcNAc. Although only approximately 2-3% of fructose-6-phosphate enters the HBP, it can reflect the cellular nutritional status and thus be used for regulatory purposes.
O-GlcNAc modification is reversible, a process known as the hexosamine cycle. The enzyme responsible for adding the sugar group (O-GlcNAcylation) is O-GlcNAc transferase (OGT), and the enzyme responsible for removing the sugar group is β-N-acetylglucosaminidase (O-GlcNAcase, OGA). Because the OGA gene was originally isolated as the meningioma-expressed antigen 5 gene, it is also known as the MGEA5 gene.
The hexosamine cycle can reversibly regulate target protein activity, acting in a manner similar to reversible phosphorylation regulation, hence the name hexosamine (O-GlcNAc) signaling pathway. This pathway is essential for survival, and defects in it often lead to severe disease or even embryonic lethality.
The hexosamine pathway is involved in nutrient responses and therefore has a significant impact on nutrient-sensitive diseases such as type 2 diabetes and neurodegenerative diseases. For example, the hexosamine pathway regulates insulin signaling and is closely linked to insulin resistance in conditions of hyperglycemia and hyperinsulinemia.
Under normal conditions, OGT is localized in the cell nucleus. However, after prolonged insulin exposure, some OGT translocates to the plasma membrane and binds to PIP3. There, OGT is activated by the insulin receptor and glycosylates several key molecules, including IRS-1, p85/p110, PDK1, AKT, and FOXO, thereby altering insulin signaling. Modification of IRS-1 alters its phosphorylation, reducing its activity and diminishing its interaction with p85, leading to decreased phosphorylation of GSK3β, FOXO, and AS160. Reduced GSK3β phosphorylation leads to decreased glycogen synthesis. Reduced FOXO phosphorylation (and increased hexosamine modification) leads to increased gluconeogenesis. Reduced AS160 phosphorylation reduces GLUT4 translocation and glucose uptake. Overall, these modifications inhibit insulin signaling.
The hexosamine pathway can also regulate processes such as apoptosis and cell division. For example, O-GlcNAc modification of p53 at Ser149 prevents phosphorylation at Thr155, inhibits its ubiquitination and degradation, and enhances p53 stability. It has been reported that hexosamine modification of p53 promotes apoptosis in retinal pericytes under hyperglycemic conditions, which is associated with diabetic retinopathy.
O-GlcNAc modification can also regulate cell division by modulating the phosphorylation of calmodulin-dependent protein kinase IV (CaMKIV). In its basal state, CaMKIV is modified with O-GlcNAc at Ser189. However, upon stimulation with ionomycin, Ser189 O-GlcNAc is reduced, significantly increasing Thr200 phosphorylation and thus becoming activated.
O-GlcNAc modification can also regulate oxidative stress signaling pathways, participate in epigenetic regulation of gene expression, and contribute to pathological processes such as tumors.