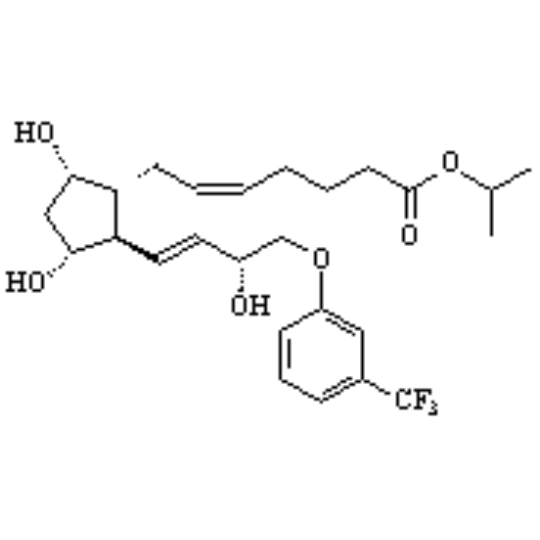
CAS of Travoprost
- CAS No.:157283-68-6
- Formula:C26H35F3O6
- Molecular Weight:500.55
- Chemical purity:≥99.0%
- Synonym:15(S)-FLUPROSTENOL ISOPROPYL ESTER; 5-Heptenoic acid; Trevostaglandin; Travoprost Eye Drops; Travoprost(Fluprostenol Isopropyl Ester); Trivo; Trivoprost; (+)-FLUPROSTENOL ISOPROPYL ESTER; FLUPROSTENOL ISOPROPYL ESTER; FLU-IPR; AL 6221; Travaprost; Travatan; TRAVOPROST; propan-2-yl ; Travoprost (For eye drops); cGMP Travoprost; propan-2-yl ;Travoprost, (±)-16-(m-Trifluoromethylphenoxy)tetranorprostaglandin F2; Travoprostintermediates;Travoprost (AL-6221); Travoprost, >=99%; Travoprost Impurity 33; Travoprost USP/EP/BP; Trovoprost;isopropyl ; Travoprost D7; Travoprost (COLD SHIPMENT REQUIRED) (1673001); ravopros; Travoprostt;Travoprost API; Isopropyl ; Travoprost, 10 mM in DMSO; Quvoprost/Quvoprost;
What is Travoprost?
Travoprost is a novel prostaglandin (PGF2α) analog. It has high selectivity and affinity for the PGF2α receptor and is a full agonist. It is used to treat primary open-angle glaucoma and ocular hypertension.
Highassay provides Travoprost API as a colorless or light yellow oil. It meets USP specifications and has a 99% purity.

Highassay is one of the major suppliers of Travoprost API in China
Highassay is one of the major suppliers of travoprost API in China, providing GMP-compliant travoprost raw materials. Our products have been sold to numerous eye drop pharmaceutical companies.
We currently offer both lab-grade and GMP-grade grades. Lab-grade fully supports domestic and international drug development. GMP-grade supports commercial production and can provide relevant registration documents.
How Does Travoprost Work?
Travoprost is a prostaglandin analog. As a selective FP1 receptor agonist, it lowers intraocular pressure (IOP) with relatively few side effects. It is primarily used to treat open-angle glaucoma and ocular hypertension. It works by increasing aqueous humor outflow through the uveoscleral pathway, thereby lowering IOP and protecting the optic nerve from damage. In clinical practice, travoprost is typically manufactured as travoprost eye drops for the treatment of open-angle glaucoma and ocular hypertension, aiming to slow disease progression and protect patients’ vision.
Highassay offers both lab-grade and GMP-grade grades, with purity levels of 97%, 98%, and 99%. Lab-grade fully supports domestic and international drug development. GMP-grade supports commercial production and can provide relevant registration documents.
Highassay is commercially producing travoprost, ensuring stable production and year-round inventory.
Yes, please contact our staff for details.
We use ice packs inside the packaging to keep the product cool. We use sealed foam boxes outside to prevent the internal temperature from being affected by the outside air. We use express delivery services such as FedEx to minimize shipping times and ensure timely delivery.
For long-term storage, refrigeration is recommended to ensure stable quality.
Specification of Travoprost
| Items | USP Standards | Results |
| Appearance | Colorless or light yellow oil | Colorless oil |
| Identification | TLC HPLC | Conforms |
| Specific rotation (20mg/ml,EtOH,365nm) | [ ɑ]D20:+52.0°~+58.0° | +56.0 ° |
| Water | 1.0% max | 0.62% |
| Limit of Ethyl acetate(GC) | 0.5%max | N.D |
| Related substance (HPLC)
| Travoprost Related Compound A: ≤0.2% Epoxide derivative: ≤0.4⁹% 15-epi Diastereomer: ≤0.1% 5,6-trans Isomer:≤3.50% 15-Keto derivative:≤0.3% Any other impurity: ≤0.1% Total impurities:≤4.0% | N.D 0.01% 0.01% 0.03% 0.15% 0.02% 0.20% |
| Assay (Anhydrous and solvents free basis) | 96.0-102.0% | 99.9% |