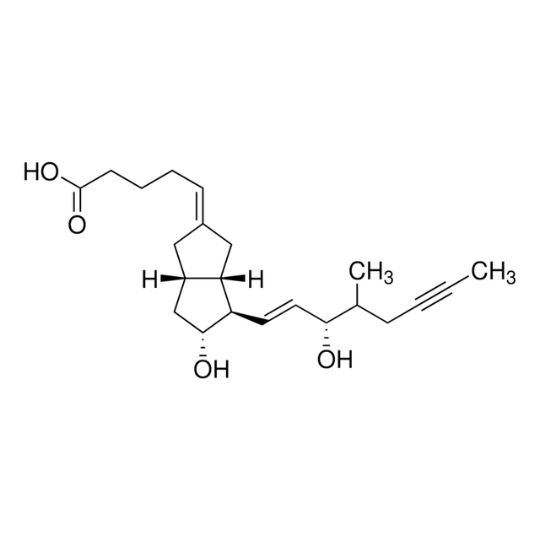
Basic information of Iloprost
- CAS No.:78919-13-8
- Formula:C22H32O4
- Molecular Weight:360.49
- Chemical purity:≥99.0%
- Synonym:LLOPROST; zk36374; Endoprost; Ilomedin; iloprostaglandin; Iloprost Na; CILOPROST; Iloprost (Ciloprost); Iloprost; Prostaglandin (PGI2) analog
Physical and Chemical Properties of Iloprost
Density: 1.21 g/cm³
Boiling Point: 539.2°C at 760 mmHg
Flash Point: 294°C
Refractive Index: 1.629
Storage Conditions: Desiccate at -20°C

Brief Introduction of Iloprost
Iloprost is a synthetic prostaglandin analog. In its pure form, it is a colorless to slightly yellow oily liquid.
It requires long-term storage at -20°C to maintain stable quality.
Highassay provides Iloprost pharmaceutical raw materials. These products meet or even exceed EP standards, currently achieving a purity exceeding 99%, with stable quality and yield. We maintain inventory year-round.
Dosage Forms of Iloprost
Iloprost finished formulations are used to treat pulmonary hypertension, cardiovascular disease, and frostbite. There are two common finished formulations: an inhalable spray for pulmonary hypertension, such as Ventavis and Waymade.
An injectable solution, such as Ilomedin, is generally used to treat frostbite.

Tech Support for Iloprost
Highassay can provide a range of support, including technical and documentation support, such as stability data sheets, residual solution test sheets, impurity testing, and endotoxin testing. Registration documents include DMF files.
Please contact us for more information.
We offer lab grade, non-steril GMP grade, and sterile GMP grade. Purity 98% min
Non-sterile product delivery is 3-5 days, and sterile product delivery is 2-3 weeks.
We typically ship by express delivery.
We offer long-term, stable supply.
We offer price discounts for bulk purchases. Please contact us for details.
Our team supports relevant registration documents for end users.
Sufficient ice packs are placed inside the packaging, and the packaging is sealed with foam packaging to ensure the product is transported at a low temperature.
Specification of Iloprost
| Tests | Standards | Results |
| Appearance | Colorless to yellowish oil | Colorless oil |
| Solubility | Freely soluble in alcohol, practically insoluble in water | Conforms |
| Identification | IR HPLC | Conforms |
| Specific Optical Rotation
| +93°~+102° | +98.2° |
| PURITY (HPLC) | 97.5%min | 99.9% |
| Impurity A | 2.0%max | ND |
| Impurity B | 1.0%max | ND |
| Impurity C | 1.0%max | ND |
| Single unknown impurity | 0.5%max | 0.06% |
| Total impurities | 2.5% max | 0.1% |
| Residual solvents | ||
| Ethyl acetate | 5000ppm max | 354ppm |
| N-Heptane | 5000ppm max | 342ppm |
| Methanol | 3000ppm max | 160ppm |
| Tetrahydrofuran | 720ppm max | ND |
| Dichloromethane | 600ppm max | ND |
| Toluenen | 890ppm max | ND |
| Loss on drying | 2.0%max | 0.23% |
| Sulfated ash | 0.1%max | 0.03% |
| Heavy metals | 20ppm max | 0.2ppm |
| Bacterial Endotoxin | 100EU/mg max | Conforms |
| Sterility | Comply with the test for sterility | Confomrs |
| Content (anhydrous basis) | 95.0-103.0% | 98.9% |