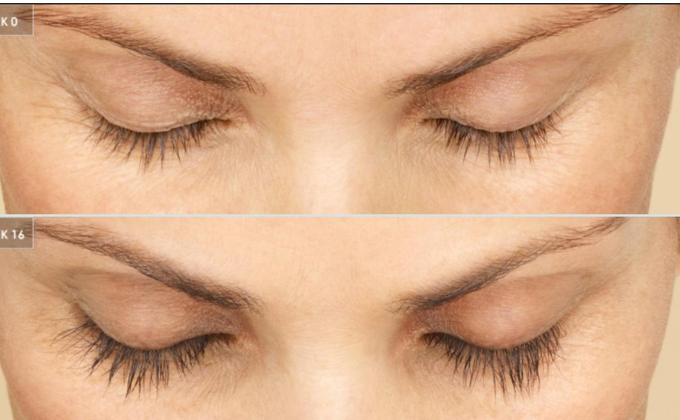
Basic information of Bimatoprost API
CAS No.:155206-00-1
Formula:C25H37NO4
Molecular Weight:415.56
Chemical purity:≥99%
Synonym:LUMIGAN; AGN 192024;Prostamide;17-phenyl-tri-norprostaglandin f2α-ethyl;
BiMapnost;
N-ETHYL-9ALPHA,11ALPHA,15S-TRIHYDROXY-17-PHENYL-18,19,20-TRINOR-PROSTA-5Z, 13E-DIEN-1-AMIDE;
What is Bimatoprost?
Bimatoprost is a synthetic structural analog of natural prostaglandins. The highly purified API is a white to almost white powder. It has two main applications: one is to treat glaucoma, reducing intraocular pressure in patients with open-angle glaucoma and ocular hypertension who are intolerant or insensitive to other intraocular pressure-lowering agents. The other is to treat insufficient eyelash hair, promoting eyelash growth, including length, thickness, and depth.

Advantages of Highassay Bimatoprost API Powder

Commercially produced, high-standard APIs comply with GMP requirements. Bimatoprost finished products contain few impurities and offer consistent and reliable quality.

Having overcome multiple synthetic difficulties, the product’s advanced process and quality standards meet the diverse needs of multiple customers.
Highassay has built a high-end prostaglandin series industrialization platform with strong market competitiveness.
Applications of Bimatoprost
Common brands of bimatoprost include Lumigan ophthalmic solution 0.01% and Somerset ophthalmic solution eye drops (0.03%-2.5ml). These are primarily used to treat intraocular hypertension.
Latisse ophthalmic solution 0.03% 5ml from the United States is used to treat insufficient eyelash hair. 3. Regarding the much-discussed hair loss treatment function, there is currently no universal data to support this claim.
Treatment of glaucoma and promotion of eyelash growth.
Non-sterile products have a delivery time of one week.
We typically use express delivery methods such as FedEx.
Highassay already commercially produces Bimatoprost on a regular basis, with year-round inventory.
We offer discounts for bulk purchases. Please contact us for details.
Specification of Bimatoprost
| Items | Standards | Results |
| Description | A white to almost white powder | Conforms |
| Identification | HPLC IR | Conforms |
| Solubility | Diffluent in acetonitrile, methanol, ethanol and acetone, soluble in dichloromethane, ethyl acetate and ether, slightly soluble in water | Conforms |
| Specific optical rotation | +33.0 ~ +37.0 o | +35.4 o |
| Residue on ignition | 0.5% max | 0.06% |
| Heavy metals | 10ppm max | 2ppm |
| Water | 1.0% max | 0.66% |
| Related substances | 15S-Bimatoprost: 0.30% max Bimatoprost free acid: 0.10% max 5,6-trans-Bimatoprost: 0.50% max Any other single impurity: 0.10% max Total other impurities: 0.50% max | 0.00 0.00 0.00 0.00
0.00 |
| Residual solvents | Acetonitrile : 0.04% max Ethanol : 0.5% max n-Hexane: 0.05% max Ethyl acetate: 0.4% max | N.D N.D N.D 0.02% |
| Assay (anhydrous and solvent free basis) | 96.0-102.0% | 99.9% |